" Mycotoxin analyses (Midwest Laboratories Inc, Omaha, Nebraska, US) showed 2.08 ppb total aflatoxins and 3.0 ppm total fumonisins in a pooled sample of the GM feed and no aflatoxins and 1.2 ppm total fumonisins in a pooled sample of the non-GM feed. No other mycotoxins were detected. These levels are well below the USA and EU limits for mycotoxins in pig feed. In addition, according to common industry practice, a mycotoxin binding agent (200 mesh bentonite clay) was added to the diets of young pigs (Table 1)."
Statement in "A long-term toxicology study on pigs fed a combined genetically modified (GM) soy and
GM maize diet" Judy A. Carman, Howard R. Vlieger, Larry J. Ver Steeg, Verlyn E. Sneller, Garth W. Robinson, Catherine A. Clinch-Jones, Julie I. Haynes, John W. Edwards
Journal of Organic Systems, 8(1), 2013, pages 38-54.
A colleague of the Pundit's who is an expert on the animal husbandry of pigs with many years experience has kindly pointed out that the assumption of Carman et al 2013 quoted above --- to the effect that measured fumonisin levels of 3 mg per kilogram (3 ppm) in the diets in their study diets are irrelevant to toxic effects -- is probably wrong.
The colleague has nicely provided to the Pundit the paper cited below by James E. Delgado and Jeffrey D. Wolt.
This paper flags the level at which fumonisin fungal toxin has toxic effects when present chronically in the diet is at about 1 mg per kilogram of diet.
This implies that the tolerance level for fumonisin laid down by American and European limits on fumonisin in feed is not sufficient to prevent some toxic effect with chronic exposure -- and it also implies that Carman et al 2013 were wrong to assume the higher level of fumonisin mycotoxin they found in their "GM-diet" compared to the level they saw in the "Non-GM" diet was unimportant as far as toxic effect.
Quite possibly the pig feed mycotoxin limits are a pragmatic compromise with what is economically feasible in terms of workable feed availability given of the widespread occurrence of this fungal toxin in stock feeds.
In simple terms, these feeds have unintentional low-level chronic toxicity due to low level mouldiness.
The pair of diets fed in the system study by Carman and others are also unrepresentative in terms of relative mould toxin content. They are the reverse of normal industry finding.
The standard industry finding is that GM insect protected grains have lower levels of mycotoxin fumonisin than non-GM grains (e.g. see below).
In the Carman and colleagues study in contrast , the pig feeds that were GM had higher levels of mycotoxin than their non-GM diet, and this unrepresentative difference may well be relevant to interpreting the study.
Surprisingly then, Carman and colleagues results, correctly interpreted and reconcilled with Delgado and Wolt 2011 could mean GM diets for pigs are generally better than non GM diets.
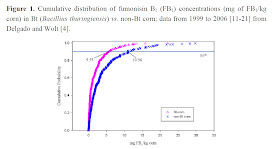 |
| Delgado and Wolt estimate Bt GM corn (pink) is more frequently lower in fumonisin toxin the not Bt corn |
Fumonisin B1 Toxicity in Grower-Finisher Pigs: A Comparative Analysis of Genetically Engineered Bt Corn and non-Bt Corn by Using Quantitative Dietary Exposure Assessment Modeling
James E. Delgado* and Jeffrey D. Wolt
Interdepartmental Toxicology Program, Department of Agronomy, Iowa State University, Ames, IA 50011, USA
Abstract: In this study, we investigate the long-term exposure (20 weeks) to fumonisin B1 (FB1) in grower-finisher pigs by conducting a quantitative exposure assessment (QEA). Our analytical approach involved both deterministic and semi-stochastic modeling for dietary comparative analyses of FB1 exposures originating from genetically engineered Bacillus thuringiensis (Bt)-corn [GM corn], conventional non-Bt corn and distiller’s dried grains with solubles (DDGS) derived from Bt and/or non-Bt corn. Results from both deterministic and semi-stochastic demonstrated a distinct difference of FB1 toxicity in feed between Bt corn and non-Bt corn. Semi-stochastic results predicted the lowest FB1 exposure for Bt grain with a mean of 1.5 mg FB1/kg diet and the highest FB1 exposure for a diet consisting of non-Bt grain and non-Bt DDGS with a mean of 7.87 mg FB1/kg diet;
the chronic toxicological incipient level of concern is 1.0 mg of FB1/kg of diet. Deterministic results closely mirrored but tended to slightly under predict the mean result for the semi-stochastic analysis. This novel comparative QEA model reveals that diet scenarios where the source of grain is derived from Bt corn presents less potential to induce FB1 toxicity than diets containing non-Bt corn.
Keywords: Bacillius thuringiensis corn; Bt corn; swine diet; DDGS; fumonisin; risk assessment
Int. J. Environ. Res. Public Health 2011, 8(8), 3179-3190; doi:10.3390/ijerph8083179
Free access @ IJERPH | Free Full-Text | Fumonisin B1 Toxicity in Grower-Finisher Pigs: A Comparative Analysis of Genetically Engineered Bt Corn and non-Bt Corn by Using Quantitative Dietary Exposure Assessment Modeling:
Update
Fumonisin B(1) and implications in nursery swine productivity: a quantitative exposure assessment.
Abstract
This study estimated the long-term exposure of fumonisin B(1) (FB(1)) in nursery swine diets and associated toxicological adverse effects on negative productivity potential using quantitative exposure assessment. Fumonisin B(1) is a mycotoxin produced by Fusarium verticillioides and Fusarium proliferatum and is a common biological contaminant of corn (Zea mays L.) and other grains. Acute effects from FB(1) exposures are well recognized and managed in the swine industry, but practices to limit prolonged low-dose exposures to FB(1) have been less fully considered and may negatively affect production efficiency. Deterministic (single-point estimates) and stochastic (probabilistic) modeling were performed for comparative analyses of FB(1) exposures originating from genetically engineered Bacillus thuringiensis (Bt)-corn, conventional non-Bt corn, and distillers dried grains with solubles (DDGS). Six feeding scenarios differing in the source of corn in diets were modeled to assess variation in FB(1) exposure representing a mixture of 1) Bt and non-Bt grain and DDGS (blended); 2) Bt grain and Bt DDGS; 3) non-Bt grain and non-Bt DDGS; 4) Bt and non-Bt grain; 5) Bt grain; and 6) non-Bt grain. Long-term exposure estimates (49-d duration) were compared with chronic levels of concern (LOC).
The first LOC (LOC1; 1 mg of FB(1)/kg of diet, least observed adverse effects concentration) represents a decrease in ADG. Concentrations of 5 mg of FB(1)/kg of diet represent the second LOC (LOC2), which showed pulmonary pathological alterations and a significant dose-dependent increase in pulmonary weight. Estimates indicated LOC1 was frequently exceeded regardless of feeding scenario, but LOC2 was not attained. Diets where the corn fraction was entirely from Bt-corn showed the least FB(1) exposure (exceeding LOC1 in 35% of occasions), whereas a blended diet or diets using non-Bt grain and DDGS sources more commonly exceeded this threshold (95% of occasions). Based on these estimates, under blended corn source feeding conditions, swine populations in nursery facilities may frequently exhibit incipient effects (i.e., LOC1) of FB(1) toxicity; however, impacts on production efficiency remain uncertain.
Delgado JE, Wolt JD. J Anim Sci. 2010 Nov;88(11):3767-77. doi: 10.2527/jas.2009-2422.
Epub 2010 Jul 9.
 |
| Delgado and Wolt 2019 |

No comments:
Post a Comment